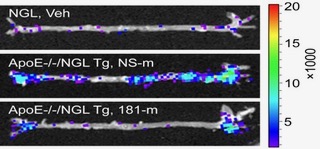
Accumulating studies reveal an important role for inflammation of the vessel wall as a major contributor in the development of a range of vascular disease states including atherosclerosis, transplantation arteriosclerosis, insulin resistance/diabetes/obesity, and thrombosis. We have identified and examined key microRNAs (e.g. miR-181b) and transcription factors (e.g. KLF10) as critical regulators of pro-inflammatory pathways such as NF-kB signaling or TGF-b1/Smad signaling in the vessel wall by regulating endothelial-leukocyte interactions. These studies have revealed new targets for anti-inflammatory therapy applicable to a range of acute and chronic inflammatory disease states.
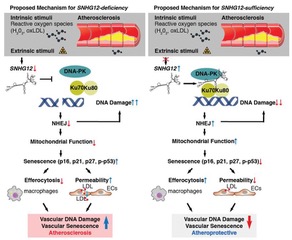
We have identified long non-coding RNAs (lncRNAs) involved in the DNA damage response and senescence in the vessel wall. We have designed delivery platforms capable of inhibiting or overexpressing these lncRNAs for therapeutic intervention. Our published and ongoing studies indicate the ability to “re-set” aging/senescence in the vasculature with implications for atherosclerosis, diabetes, and repair of injury in ischemic tissues
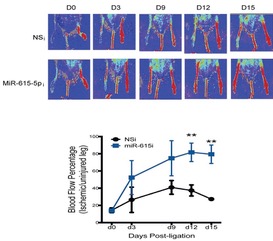
The induction and orchestration of new blood vessels is critical for tissue repair in response to injury in ischemic cardiovascular diseases such as myocardial infarction (MI) or peripheral artery disease (PAD). Subjects with diabetes develop higher rates of cardiovascular complications in part due to impaired microvascular disease. Using a variety of profiling approaches, we have identified microRNAs (e.g. miR-26a, miR-615-5p, miR-135-3p), lncRNAs, transcription factors (e.g. KLF10), and signaling pathways (e.g. TGF-β1/BMP) that improve neovascularization after myocardial or limb ischemia. The results of these studies provide the foundation for novel therapies directed at the treatment of a broad spectrum of ischemic cardiovascular disorders.